Under Which Conditions Will Gases Best Dissolve in Liquids
The dissolving of a gas in water depends on the interaction between the molecules of the gas and the water molecules. Decreased A gass solubility is faster in a liquid when under high low pressure.
But gases are very dependent on the pressure of the system.

. For example the equilibrium between oxygen gas and dissolved oxygen in water is O 2 aq O 2 g. The gas molecules in a liquid are dissolved by the process of dissolution. The contrast at the microscopic level between solids liquids and gases is most clearly seen in the simplified schematic views above.
Lowering the temperature of a liquid decreases. On the other hand the solubility of a gas in a liquid increases with the pressure exerted. The kind of solute that dissolves best under high temperature conditions would be a solid What kind of solute solid liquid or gas dissolves best under high temperature conditions.
See full answer below. You can say that in the first case it is pushed into the liquid and in the latter more slower moving molecules are happy to be restricted to less movem. Colder water will be able to have more gas dissolved in it.
A gas is most soluble in water under conditions of high pressure and low temperature. What are the best conditions for dissolving a gas in a liquid. There are several molecular reasons for the change in solubility of gases with increasing temperature which is why there is no one trend independent of gas and solvent for whether gases will become more or less soluble with.
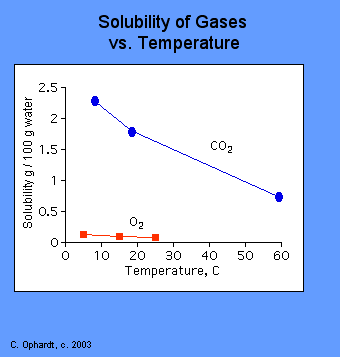
The amount of gas that can be dissolved in water depends on the temperature of the water. Solubilities of Gases in Water Methane oxygen carbon monoxide nitrogen and helium all have different solubilities in water but all of them become less soluble with increasing temperature. High pressure to force the gas into the liquid and low temperature so that the moving gas particles have less resistance to.
The molecular units of crystalline solids tend to be highly ordered with. Under which set of conditions will the unknown gas XY be most soluble in a liquid. Under which conditions will gases best dissolve in liquids.
If the pressure is increased the gas molecules are forced into the solution since this will best relieve the pressure that has been applied. According to Le Chateliers Principle which states that when the equilibrium of a system is disturbed the system readjusts itself in such a way that the effect that has caused the change in equilibrium is countered. In the case of gases the decrease in intermolecular forces releases the gas molecules from the forces that keep them in solution and will escape from the container so it will be observed that as the temperature increases the solubility of a gas decreases in a solvent like water.
As a result the simple dissolved form is the only form that contributes to the partial pressure of the gas. Certainly a soft drink usually sealed under pressure will foam more vigorously when it is at room temperature than when it is chilled. Importantly only the simple dissolved form is in dynamic equilibrium with the traditional gas phase surrounding the liquid.
These are so short-lived however that even under extreme conditions gases cannot be said to possess structure in the usual sense. The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution. Attractive intermolecular interactions in the gas phase are essentially zero for most substances.
Gases can exist in liquids as a simple dissolved molecule bound to a protein or in a chemically-modified state. This dissolution is an equilibrium process for which an equilibrium constant can be written. Because heat is released when these new attractive interactions form dissolving most gases in liquids is an exothermic process ΔH_soln 0.
Henrys Law states that. Gases dissolve in liquids to form solutions. If a solid solute is not dissolving well in water you can increase its.
When a gas dissolves it does so because its molecules interact with solvent molecules. Chemistry 22112019 1231 kaileyy06. Usually the higher the gas pressure above the liquid and the lower the temperature the more the gas dissolves.
During this process heat is evolved. More gas can dissolve in cold water than in hot water. A gas dissolves faster in a liquid if the temperature of the liquid is increased decreased.
And you can demonstrate this every time you take the cap off a bottle of fizzy or carbonated drink. While it is in general true for gases dissolved in water gases dissolved in organic solvents tend to become more soluble with increasing temperature. XY is a polar gas dissolved in a nonpolar solvent XY is a nonpolar gas dissolved in a polar solvent XY is a polar gas dissolved in a polar solvent The.
If under enough pressure all gases can dissolve in liquids. Gases best dissolve in liquids when the liquid temperature is low and when pressure is increased. In general solubility of a gas in water will decrease with increasing temperature.

Can Gases Dissolve In Water Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
Dissolved Gases In Purified Water Elga Labwater

Gas Solubility An Overview Sciencedirect Topics
How Do Gases Dissolve In Water Quora

13 4 Solutions Of Gases In Water How Soda Pop Gets Its Fizz Chemistry Libretexts

Solubility Of Gas In Liquid Concept Pressure Temperature Video Lesson Transcript Study Com
Solubility Factors When Choosing A Solvent
20 4 Cell Potential Under Standard Conditions Chemistry Libretexts

Can Gases Dissolve In Water Chapter 5 The Water Molecule And Dissolving Middle School Chemistry
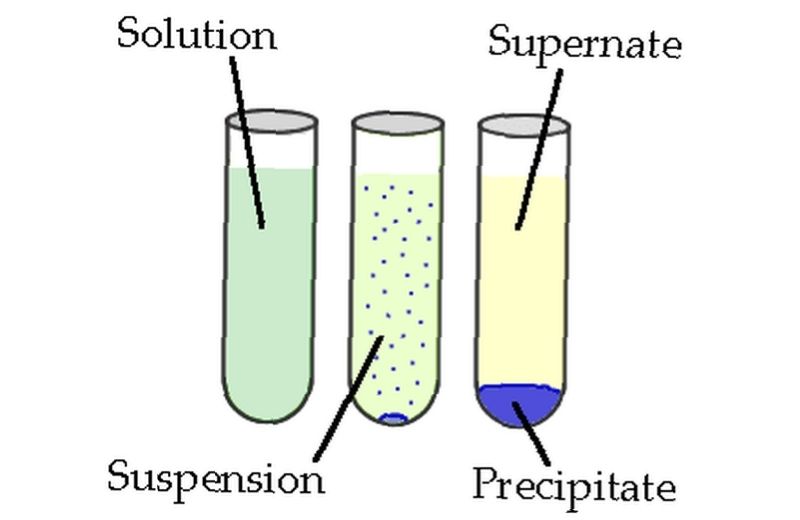
Solubility Solid In A Liquid Gas In A Liquid Liquid In A Liquid Notes

Factors Affecting Solubility Henry S Law And Rate Of Dissolving

Factors Affecting Solubility Boundless Chemistry
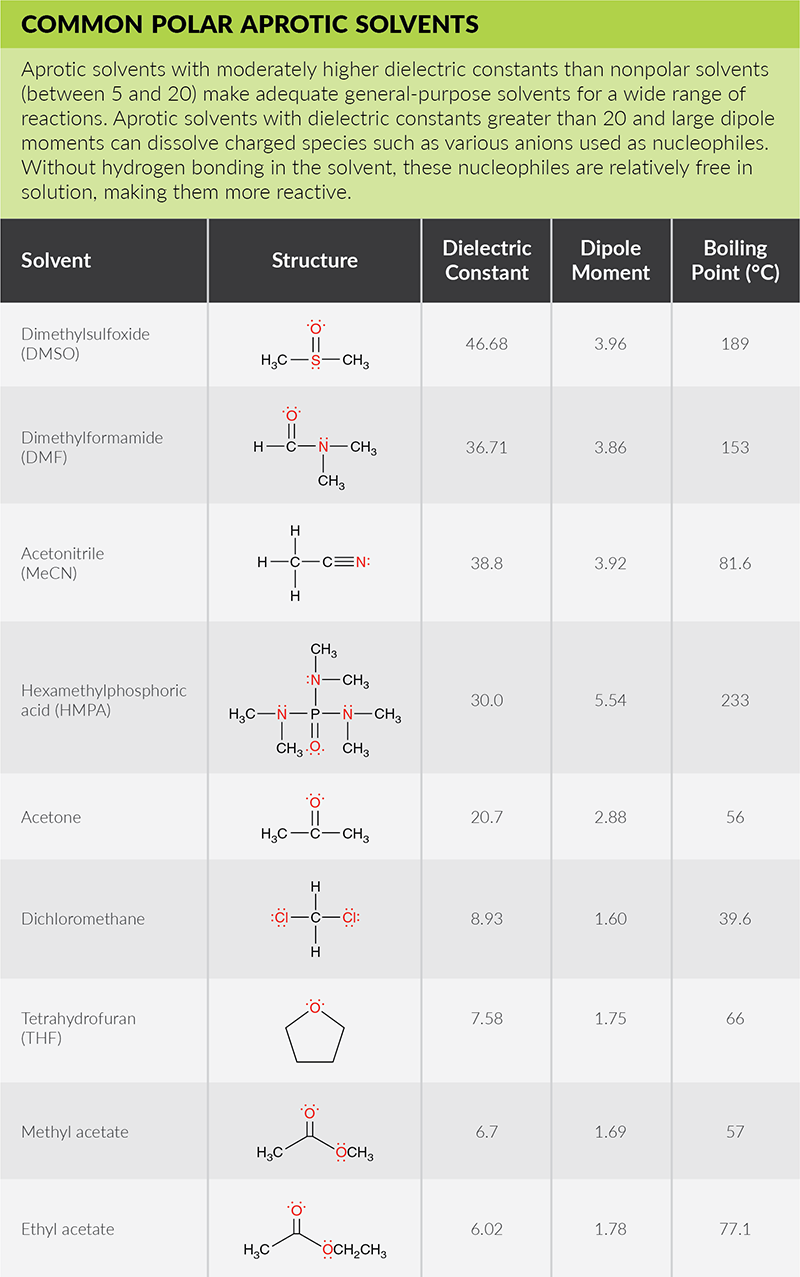
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical
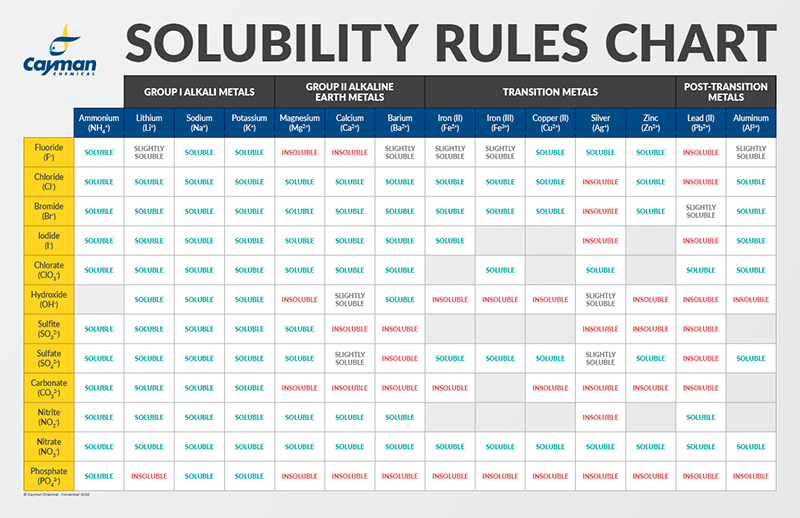
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical

Solubility Solid In A Liquid Gas In A Liquid Liquid In A Liquid Notes
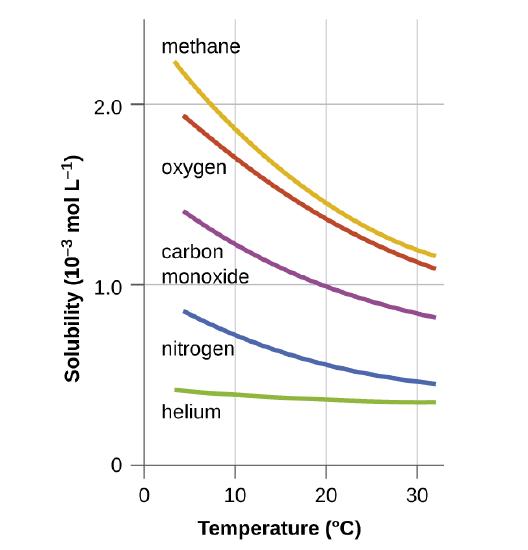
13 4 Solutions Of Gases In Water How Soda Pop Gets Its Fizz Chemistry Libretexts
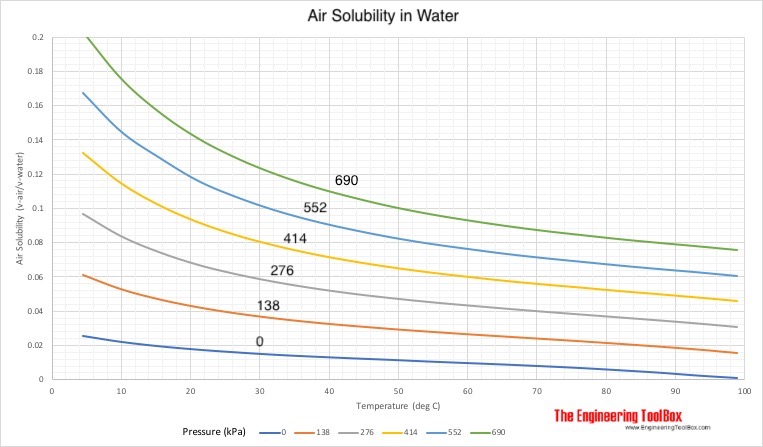
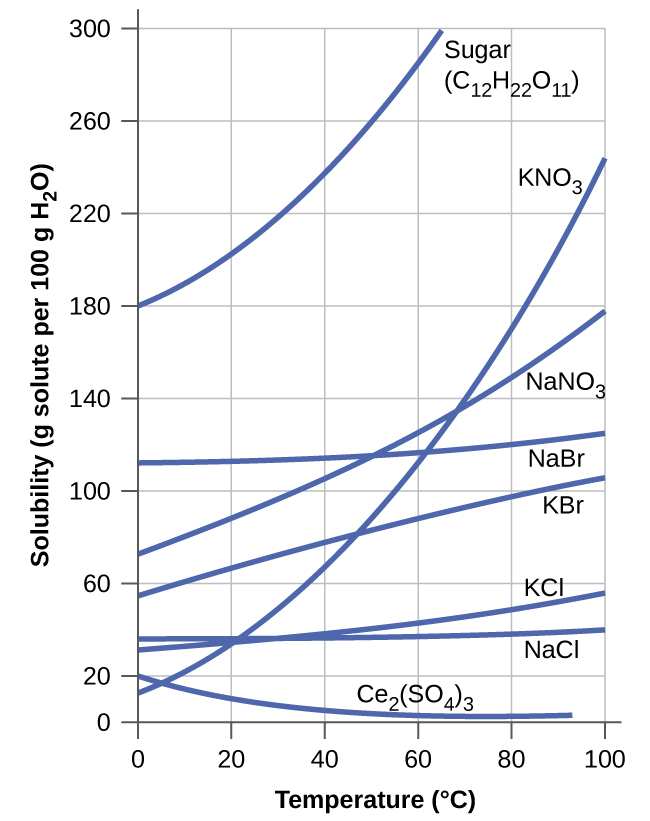

Comments
Post a Comment